Background: Despite recent advances in the treatment of B-cell non-Hodgkin lymphomas (NHL), most patients with relapsed/refractory (R/R) disease are at high risk of dying from their disease. Bispecific antibodies (BsAbs) are novel therapies with favorable response rates in R/R B-cell NHL even after cellular therapies. Mosunetuzumab, epcoritamab, glofitamab, and odronextamab are CD20 targeting BsAbs that have proven efficacy for R/R B-cell NHL. These treatments are generally well tolerated, but the infectious complications are not well described in the literature. Our study aims to quantify the relative incidence of infections associated with B-cell NHL targeted BsAbs.
Methods: We conducted a preliminary evaluation, of an ongoing pooled analysis encompassing single agent BsAbs used in B-cell NHL with no prior use of alternate BsAbs. Exclusion criteria included studies reporting the use of BsAbs as part of combination therapy and trispecific antibodies.
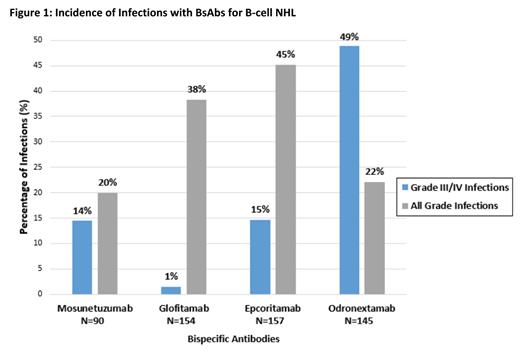
Results: A total of 546 B-cell NHL patients (number of trials, n=4) were treated with BsAbs in the studied period. Pooled median follow up was 11.7 . Relevant adverse events included all grade neutropenia in 26.9% (n=147/546), grade III/IV neutropenia in 20% (n=109/546), all grade infections in 40.1% (n=219/546), grade III/IV infections in 16.8% (n=92/546), all grade pneumonia in 7.2% (n=17/235), and coronavirus 2019 (COVID-19) in 1.3% (n=3/235). Incidence of grade III/IV and all grade infections of the BsAbs in each study are shown in figure 1. In addition, all grade cytokine release syndrome was seen in 56.4% (n=308/546) and death was reported in 33 patients of which 4 (0.7%) were reported to be secondary to infections.
Conclusion: Data from this ongoing analysis better describes the various infectious risks of patients with B-cell NHL receiving BsAbs. Providers should remain vigilant regarding these risks and intervene promptly in such situations. The finalized pooled analysis with a larger sample size may corroborate these findings.
Disclosures
Chavez:Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Genmab: Honoraria; Epizyme: Speakers Bureau; Cellectar: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra Zeneca: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Research Funding; Lilly: Honoraria; Merck: Research Funding; Morphosys: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees. Vargas Madueno:Janssen: Consultancy, Other: Advisory Board. Sandoval-Sus:Genmab: Other: Advisory Board; Janssen: Other: Advisory Board; Genentech: Other: Ad Board; BeiGene: Other: Ad Board; Abbvie: Other: Ad Board; Seagen: Other: Advisory Board, Speakers Bureau; ADC Therapeutics: Other: Advisory Board; Incyte: Other: Advisory Board; MassiveBio: Other: Advisory Board; TG Therapeutics: Other: Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal